Dr. Scott Sigman has been using BioBrace® in his practice for roughly 26 months. We sat down with him to discuss his experience with BioBrace® and its impact on his patient outcomes.
Dr. Sigman, how would you describe BioBrace®?
BioBrace® is an implant for rotator cuff, ACL surgery, and more. The concept is to enhance the quality and durability of your soft tissue repair by augmenting with this reinforced implant. It’s been shown to prevent gapping or re-tears by increasing the thickness of the tendon.1,2
What problem does BioBrace® solve?
Rotator cuff disease is not a purely mechanical problem. It is a biologic problem and a mechanical problem. Therefore, you want both of those arrows in your quiver as you’re strategizing for repair. BioBrace® addresses both.
What have you noticed in your cases since incorporating BioBrace®?
What I'm seeing has been quite unique. None of my BioBrace® cases are “softball” rotator cuff repairs. They’re all severely degenerative tears or revision tears. These are the ones that most of us would look at and say, “this is going to fail.” … yet the clinical outcomes have been remarkable.
In your opinion, what makes BioBrace® unique?
First, it's highly porous with type 1 collagen.1 Why is that important? The fibroblasts (the healing cells of the body) need to be able to migrate into this scaffold to lay down new collagen and incorporate. You need a pathway for those fibroblasts to make their way in. This provides that.
Second, it gives you strength by having these PLLA-microfilaments in the scaffold too.1 Think about rebar in concrete. You have great strength from the fibrils, but you also have the porous nature of the scaffold. This allows fibroblastic in-growth and collagen creation to facilitate healing across the tissue.
That’s why we call BioBrace® the Goldilocks scaffold.
The synthetic scaffolds out there create a really tight space… maybe there are a couple little crannies where a fibroblast could get in, but not a lot of room for motion and movement. (See below)

SYNTHETIC SCAFFOLD
+ Good Strength
-- Poor Healing
I'm also familiar with the collagen scaffold, shown below. There are no structural filaments in this scaffold. It is bioinductive but adds zero strength to your construct.

COLLAGEN SCAFFOLD
+ Good Healing
-- Poor Strength
The uniqueness of the BioBrace® implant is in the scaffold. The mechanism of infiltration of the host cells with fibroblasts, plus load sharing on Day Zero. You have immediate structure and strength for your rotator cuff, then functional remodeling.1,2 It’s also unique because you can put suture in it.

BIOBRACE® SCAFFOLD
+ Good Healing
+ Good Strength
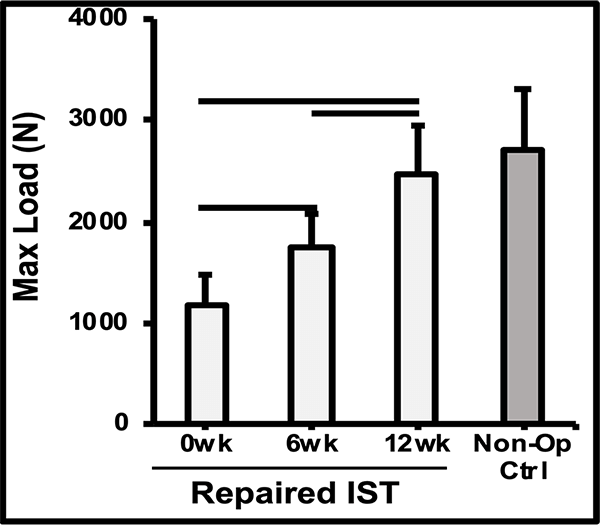
The mechanism of action was demonstrated in a preclinical sheep model, showing a thicker tendon at 6-12 weeks, and a repair that’s as strong as the native tendon by 12 weeks.1,2
MRI & Histology
Biomechanics
Can you tell us about the case study you did?
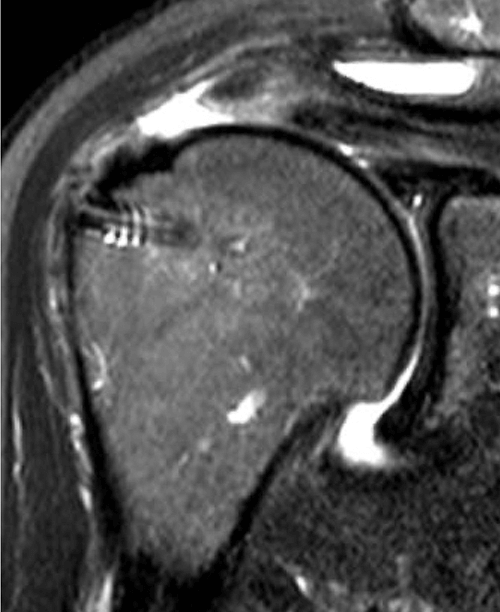
This is one of my cases, a 55-year-old woman, when her primary rotator cuff repair was done, we used Dermis on Demand, which is an allograft skin that can be utilized to help strengthen repairs. At four months out, the patient presented with pain and weakness following a traumatic injury and wound up re-tearing.
You can see a clear retear to the rotator cuff here with slight retraction, and some degeneration and thickness to the tendon.
This was not a softball case. Many aren’t. These are the complex rotator cuffs that worry you at night... Are you going to be able to fix it? Yes. But will it heal? That's the challenge. And that's where this implant helps you on a day-to-day basis.
We published this case study in the JOEI (Journal of Orthopedic Experience and Innovation) Journal. We did a revision of the large type-II repair and augmented it with BioBrace®. You can see the pre-op and three-month MRI below. Still fluid in the subacromial space but that tendon is clearly coming across into the footprint. Then, an eight-month MRI shows there's no further fluid in the subacromial space and maturation of that repair across the footprint.
Pre-OP
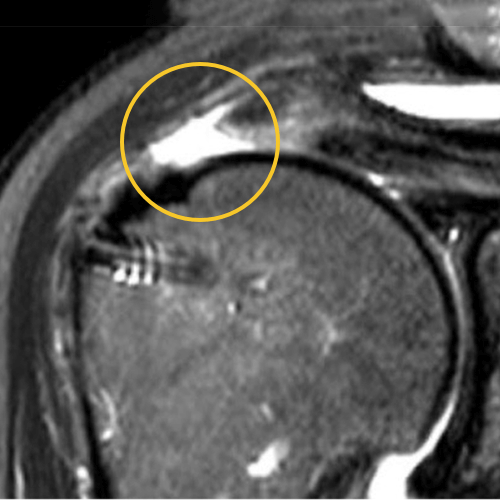
3 Months
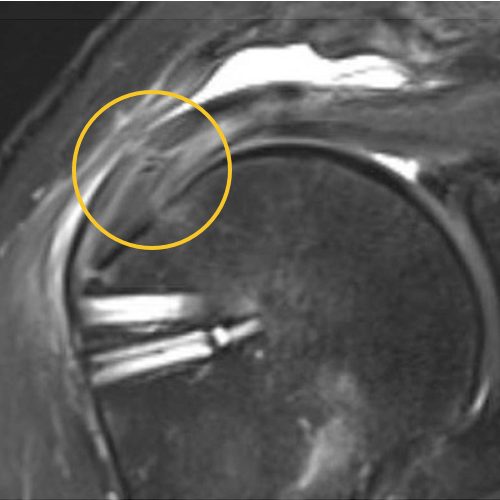
8 Months

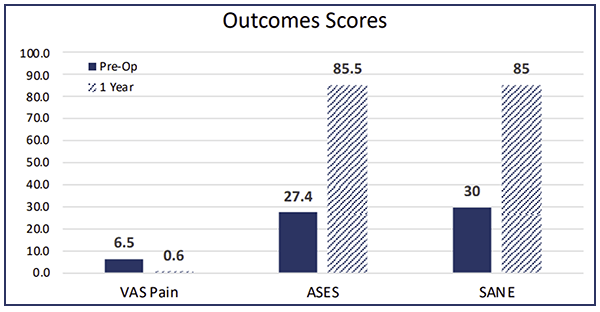
This is a patient that’s been in pain for quite some time and was clinically improving. You can see the outcome scores show dramatic improvement.
Down the road, this patient was having some bicipital pain, so we took the opportunity to look at the repair visually and performed a biceps tenodesis. You can see in this image, there's cabling of the fibers coming down onto the footprint and you can no longer see the scaffold… it’s been completely incorporated into the repair. For this patient, that was a pretty remarkable event.
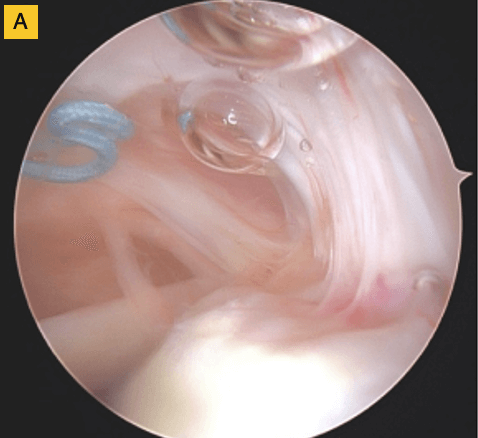
(A) Tendon fibers can be seen inserting on to footprint in an organized fashion. Visible, prominent suture easily removed.
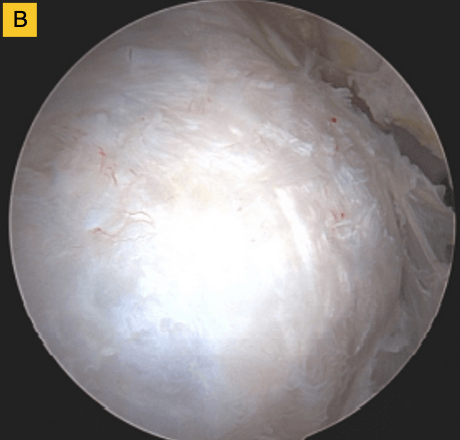
(B) BioBrace® well-synovialized and completely incorporated into the rotator cuff with new native tissue in and around the implant.
Can you describe your technique?
You can watch my full technique here. It gives you an anatomic repair of the rotator cuff on the footprint with a reinforced bioinductive strength scaffold over top of it.
There is no specific instrumentation for this implant as there are for other potential scaffolds and you can incorporate this into your standard rotator cuff repair. It’s also agnostic, so if you're a SCOI-based person and you want to do a single row repair, or if you're more traditional and you want to do a double row repair, these are all options that BioBrace® can support.
People make fun of me now, they say “Siggy you let your patients do burpees in the PACU?” Now, I think that's a little aggressive, but we do believe in early range of motion for our rotator cuff repairs.
Bottom line, with this type of a construct you can feel really comfortable that early active assisted range of motion is going to allow this patient to wind up with a solid repair.
How are the rest of your clinical results using BioBrace®?
We're up to 26 months at this point. Our patients are enrolled in a registry, and you can see the follow-up. What's really fascinating, is I am not typically using BioBrace® for those little sweetheart, one centimeter supraspinatus tears. Every single one of these patients enrolled in this study has been someone that’s had a bad tear pattern. These are hard rotator cuff repairs where you worry whether they're actually going to heal or not. You can see here, 46 patients at this point, with 26 primaries, and 23 of those are still viable and intact.

If you look at the literature for these types of tear patterns, failure rates are as high as 35–40%. These are not repairs you're typically very confident about…so these types of numbers, although early, are still very impressive.
And these repairs typically fail early. They could be failing on the way to the PACU! So when they start lasting two, three, four months and your patients are doing clinically well with functional range of motion, you should be pretty excited.
In the revision situation, which is even worse as far as the numbers are concerned, 17 patients have had revision rotator cuff repairs with BioBrace® and only two have gone on to retear. Again, pretty remarkable.
These results are very early on, and I want to be clear about that, but for these complex cases, if you're doing soft tissue repair, you're going to want this in your bag.
About BioBrace®
To date, over a thousand BioBrace® implants have been used in the United States. There are 42 unique procedures that have been performed utilizing this graft, including gluteus medius repair, shoulder rotator cuff repairs, subscapularis repairs, reverse total shoulders, AC joint reconstruction, elbow ulnar collateral ligament reconstruction, ACL, MCL, and the Achilles and collateral ligaments in the Foot and Ankle.
BioBrace® is available in two sizes and is FDA indicated to reinforce soft tissue where weakness exists..
To learn more about BioBrace®, click here.
1 K203267 – 510(k) Clearance Letter - The BioBrace® Implant
2 Based on preclinical animal data